When Is Enol More Stable Than Keto
I the hydrolysis of the phosphate ester to give the enol form of pyruvate followed by ii tautomerization to the thermodynamically stable keto form of. July 17 2015 at 154 pm.

Keto Enol Tautomerism Chemistryscore
Diketone linker present in curcumin 1 may not be necessary for the antitumor activity.

. Such isomerization is called ketoenol tautomerism. PEP hydrolysis may be formally divided into two parts. Have emerged with potential as drug candidates.
Alkynes are oxidized by the same reagents that oxidize alkenes. That is the enol from predominates. Over the past few decades more potent and more stable curcumin derivs.
Some important SAR studies pointed out that the unstable -unsatd. Sapers and Ziolkowski 1987. Q Enol formation is called enolization.
More stable forms of ascorbic acid derivatives such as erythrobic acid 2- and 3-phosphate derivatives of ascorbic acid phosphinate esters of ascorbic acid and ascorbyl-6-fatty acid esters of ascorbic acid have however been developed to overcome problems associated with ascorbic acid Sapers et al. The keto and enol forms are said to be tautomers of each other. Phenol exhibits keto-enol tautomerism with its unstable keto.
The small amount of stabilisation gained by exchanging a CC bond for a CO bond is more than offset by the large destabilisation resulting from the loss of aromaticity. The thermodynamically stable form of PEP in solution at physiological pH is the enol form. In a similar fashion the lessstable intermediate generates an aldehyde.
4 4 Substituted cyclohexadienone can undergo a dienonephenol rearrangement in acid. Mechanism of Acid-Catalyzed Enolization. That is the enol from predominates.
Disubstituted alkynes react with potassium permanganate to yield vicinal diketones Vicdiketones or 12diketones or under more vigorous conditions carboxylic. In Question 10 as six membered ring are more stable than seven membered ring due to more angle strain in seven memberedthe carbocation rearrangement results into hydrogen shift instead of ring expansionAm I right. Despite this thermodynamic driving force the enol ether described above is completely stable to base treatment and undergoes rapid acid-catalyzed hydrolysis with loss of methanol rather than.
Phenol therefore exists essentially entirely in the enol form. The interconversion of the two forms involves the movement of an alpha hydrogen atom and the reorganisation of bonding. Generally shorter linkers result in more potent compds.
Seib and Liao 1987. The preference for the carbonyl form over the enol form derives from the well-known circumstance that the CO function is imuch more stable than the CC function. The mechanism whereby enols are formed in acidic solution is a simple two step process as.
On the other hand because the endo-enol ether intermediate is more stable than the exo-isomer it is credible for the isomerization of exo-enol ether intermediate into the endo-isomer which could undergo 4 2 annulation reactions with βγ-unsaturated α-ketoesters to prepare the corresponding fused bicyclic products. Enols or more formally alkenols. The potential energy change for this rearrangement is even more advantageous than for enol-keto tautomerism being estimated at over 25 kcalmole from bond energy changes.
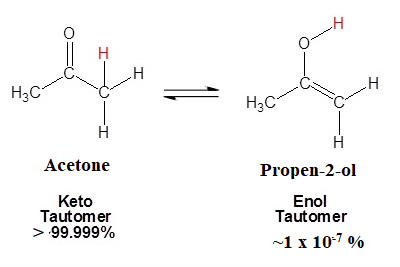
6-member rings are more stable due to the ability to adapt a chair conformation and 1095. In organic chemistry ketoenol tautomerism refers to a chemical equilibrium between a keto form a ketone or an aldehyde and an enol an alcohol.

Organic Chemistry Which Is The More Stable Enol Form Chemistry Stack Exchange


Comments
Post a Comment